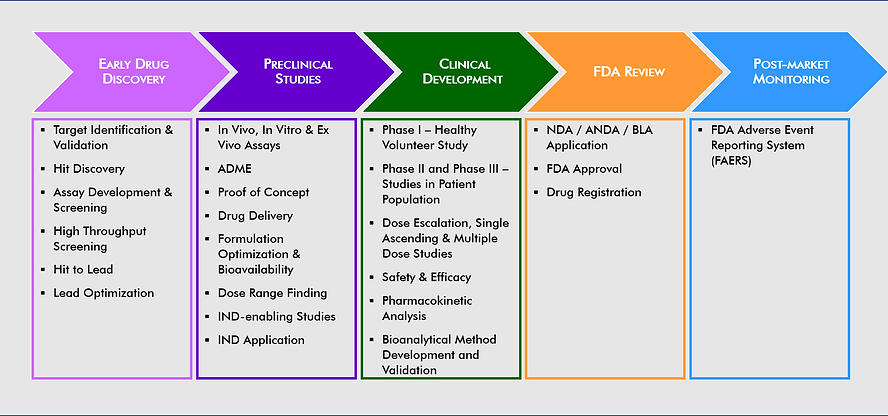
The Road to Clinical Development
The IND/CTA-enabling programme
Following lead optimisation and subsequent candidate selection during drug discovery, the Investigational New Drug (IND) moves into pre-clinical development – studies that prepare the molecule for clinical development in humans:
+ Termed IND-enabling studies – these involve primarily DMPK and more recently DDI studies along with CMC, safety pharmacology and toxicological assessment which collectively permits the initiation of a closely monitored Phase I and subsequent early Phase II clinical studies
+ In the United States, the initial submission to permit use of an investigational drug in a clinical setting is called an investigational new drug (IND) application.
+ In the European Union, this documentation is submitted within a clinical trial application (CTA).
In clinical Phase I studies, the IND is administered to humans for the first time. Early Phase I studies (sometimes termed Phase 0) relate to first-in-human (FIH) studies where a small group of subjects, usually 10 to 15 volunteers, receive a single, sub-therapeutic dose to obtain pharmacokinetic information without inducing pharmacological effects:
+ These studies typically involve 20–50 healthy volunteers (sometimes patients), If successful, further studies may assess safety and tolerance of the IND in human subjects.
The goal of phase I exploratory studies is to:
+ Investigate whether the drug presents no major safety issues
+ Determine that it can reach the targeted body area, and remain in residence long enough to deliver its benefits
+ Gain preliminary evidence that it could offer therapeutic value, or prevent the disease or condition
+ To determine early human in vivo metabolite profiles for MIST assessment and whether the drug candidate performs as expected based on preclinical studies (IVIVE).
DMPK Services can advise on the program of studies required for CTA and IND - design, cost, material resources, outsourcing process, timelines.