Drug Drug Interactions
An increasingly important aspect of new drug development is the assessment of drug-meditated drug-drug interactions (DDI).
+ As our populations are aging, polypharmacy represents a growing and significant risk factor and regulatory authorities are sensitized to this issue and have published DDI assessment guidance(s) worldwide.
+ These guidances are broadly similar across FDA, EMA and PMDA [click here for links] and focus on how new chemical entities should be tested for interactions with both drug metabolizing enzymes such as CYP and UGT and also drug transporters using in vitro methods.
+ New chemical entities should be tested as both potential perpetrator and victim of DDIs using pre-scribed in vitro cut-offs (R values) - triggering of these cut-off ratios may result in a clinical assessment of the DDI parameter affected being advised.
+ For victim drugs (high Fm), therapeutic window is crucial as perpetrator effects essentially 'create' fast/poor metaboliser phenotypes. A large therapeutic window helps to negate regulatory concern.

PM/IM/RM/URM - Poor/Internediate/Rapid/Ultrarapid metaboliser phenotpye
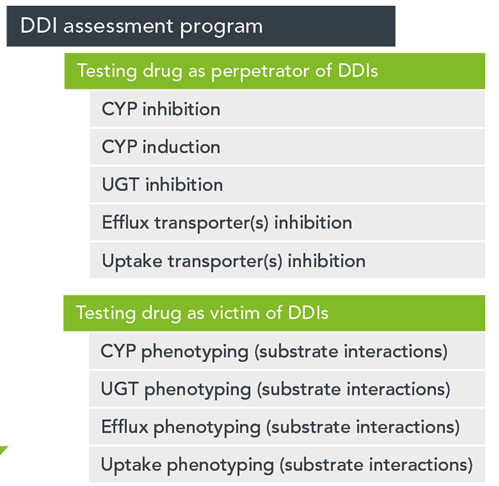
The in vitro assessments, largely conducted using using human liver microsomes and/or human hepatocytes and recombinant transporter proteins are listed below.
NOTE - since the issue of their current DDI guidance (Jan 2020), the FDA recommend these in vitro studies should be conducted before clinical development begins and therefore DDI assessment should now be included in the IND enabling program.